Welcome to PrintableAlphabet.net, your best source for all things related to Are Weaker Bases More Stable In this extensive overview, we'll look into the intricacies of Are Weaker Bases More Stable, providing beneficial understandings, involving tasks, and printable worksheets to boost your understanding experience.
Understanding Are Weaker Bases More Stable
In this area, we'll explore the fundamental concepts of Are Weaker Bases More Stable. Whether you're an instructor, moms and dad, or student, getting a strong understanding of Are Weaker Bases More Stable is vital for successful language acquisition. Anticipate understandings, tips, and real-world applications to make Are Weaker Bases More Stable revived.
List Of Common Strong And Weak Acids
:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
Are Weaker Bases More Stable
The key to understanding this trend is to consider the hypothetical conjugate base in each case the more stable weaker the conjugate base the stronger the acid Look at where the negative charge
Discover the relevance of grasping Are Weaker Bases More Stable in the context of language growth. We'll go over exactly how effectiveness in Are Weaker Bases More Stable lays the structure for improved reading, writing, and total language abilities. Check out the more comprehensive influence of Are Weaker Bases More Stable on effective interaction.
Solved 7 Aromatic Amines Are Much Weaker Bases Than Chegg

Solved 7 Aromatic Amines Are Much Weaker Bases Than Chegg
Strong acids readily give up their protons because the resulting conjugate base becomes more stable with respect to its new electron distribution An example of this would
Learning does not need to be dull. In this area, discover a range of engaging activities customized to Are Weaker Bases More Stable learners of every ages. From interactive games to imaginative workouts, these tasks are created to make Are Weaker Bases More Stable both fun and educational.
I Tapped Into The Collective Energy Just To Get An Idea Of The Overall

I Tapped Into The Collective Energy Just To Get An Idea Of The Overall
However the conjugate base of phenol is stabilized by the resonance effect with four more resonance contributors and the negative is delocalized on the benzene ring so the conjugate base of phenol is much
Access our specially curated collection of printable worksheets focused on Are Weaker Bases More Stable These worksheets deal with various skill degrees, ensuring a personalized learning experience. Download and install, print, and take pleasure in hands-on activities that enhance Are Weaker Bases More Stable abilities in an efficient and pleasurable method.
20 Marvel Characters That Are Weaker Than Fans Think CBR

20 Marvel Characters That Are Weaker Than Fans Think CBR
These are very much weaker bases than ammonia Explaining the differences in base strengths The factors to consider Two of the factors which influence the strength of a base are the ease with which the lone pair picks up a hydrogen
Whether you're a teacher looking for effective approaches or a student looking for self-guided methods, this area offers functional tips for understanding Are Weaker Bases More Stable. Take advantage of the experience and understandings of educators who concentrate on Are Weaker Bases More Stable education and learning.
Connect with like-minded people that share an enthusiasm for Are Weaker Bases More Stable. Our community is a space for educators, moms and dads, and students to trade concepts, seek advice, and celebrate successes in the journey of grasping the alphabet. Sign up with the conversation and belong of our expanding area.
Get More Are Weaker Bases More Stable





:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png?w=186)
https://chem.libretexts.org/Bookshelves/…
The key to understanding this trend is to consider the hypothetical conjugate base in each case the more stable weaker the conjugate base the stronger the acid Look at where the negative charge

https://chemistry.stackexchange.com/questions/10650
Strong acids readily give up their protons because the resulting conjugate base becomes more stable with respect to its new electron distribution An example of this would
The key to understanding this trend is to consider the hypothetical conjugate base in each case the more stable weaker the conjugate base the stronger the acid Look at where the negative charge
Strong acids readily give up their protons because the resulting conjugate base becomes more stable with respect to its new electron distribution An example of this would

SpacedNikki On Twitter Some More Stable Defusions I ve Made For The

Fe 3 Is Stable Than Fe 2 The Reason Is are

JE6WHX On Twitter RT tuftelndotnet A Project In The Works Want

A More Stable Canada U S Relationship But Let s Not Lose Momentum On
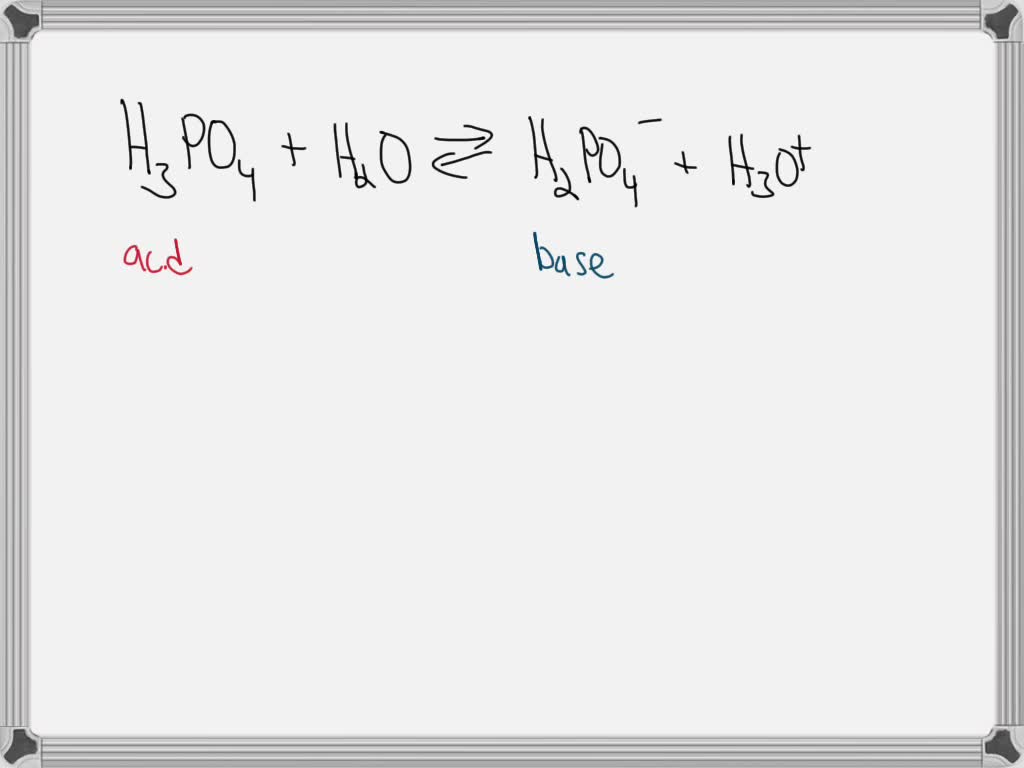
SOLVED For The Following Equilibrium Label The Stronger And Weaker
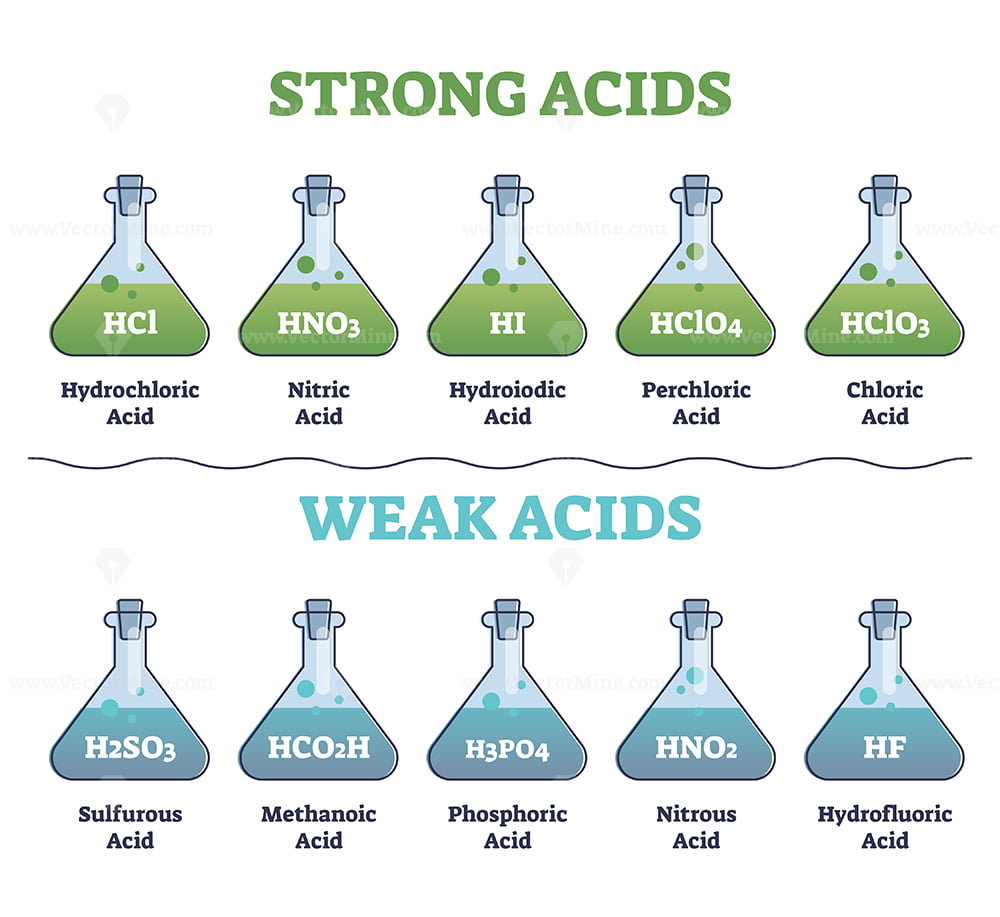
Strong And Weak Acids Collection With Educational Diagram Outline

Strong And Weak Acids Collection With Educational Diagram Outline

PDF Stabilizing Forwards For A More Stable Market
