Welcome to PrintableAlphabet.net, your go-to resource for all things related to What Does Weaker Acid Mean In this extensive overview, we'll explore the ins and outs of What Does Weaker Acid Mean, providing beneficial understandings, involving activities, and printable worksheets to enhance your understanding experience.
Understanding What Does Weaker Acid Mean
In this section, we'll explore the fundamental principles of What Does Weaker Acid Mean. Whether you're an instructor, parent, or student, getting a solid understanding of What Does Weaker Acid Mean is important for effective language purchase. Expect understandings, suggestions, and real-world applications to make What Does Weaker Acid Mean come to life.
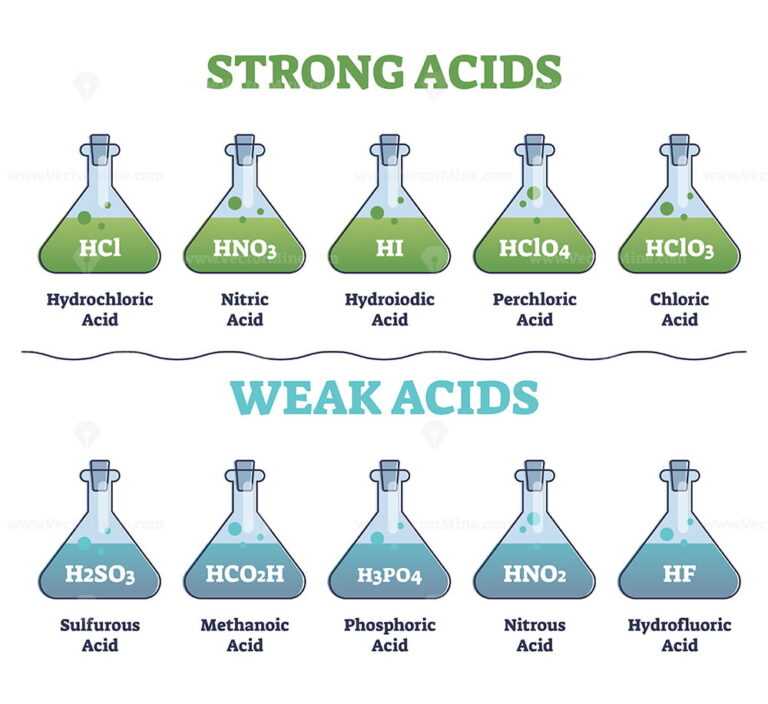
List Of Common Strong And Weak Acids
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
What Does Weaker Acid Mean
Stronger acids form weaker conjugate bases and weaker acids form stronger conjugate bases Thus strong acids are completely ionized in aqueous solution because their conjugate bases are weaker bases than water
Discover the significance of grasping What Does Weaker Acid Mean in the context of language growth. We'll talk about how proficiency in What Does Weaker Acid Mean lays the structure for enhanced analysis, creating, and total language abilities. Discover the wider influence of What Does Weaker Acid Mean on reliable communication.
How To Determine If Acid Is Strong Or Weak Shortcut W Examples And

How To Determine If Acid Is Strong Or Weak Shortcut W Examples And
A weak acid is one which doesn t ionize fully when it is dissolved in water Ethanoic acid is a typical weak acid It reacts with water to produce hydroxonium ions and ethanoate ions but the back reaction is more successful than the forward one The ions react very easily to reform the acid and the water
Understanding doesn't have to be plain. In this area, discover a range of interesting activities tailored to What Does Weaker Acid Mean students of all ages. From interactive games to innovative exercises, these activities are developed to make What Does Weaker Acid Mean both fun and academic.
List Of Strong Acids More Than 8 Examples With Images Teachoo

List Of Strong Acids More Than 8 Examples With Images Teachoo
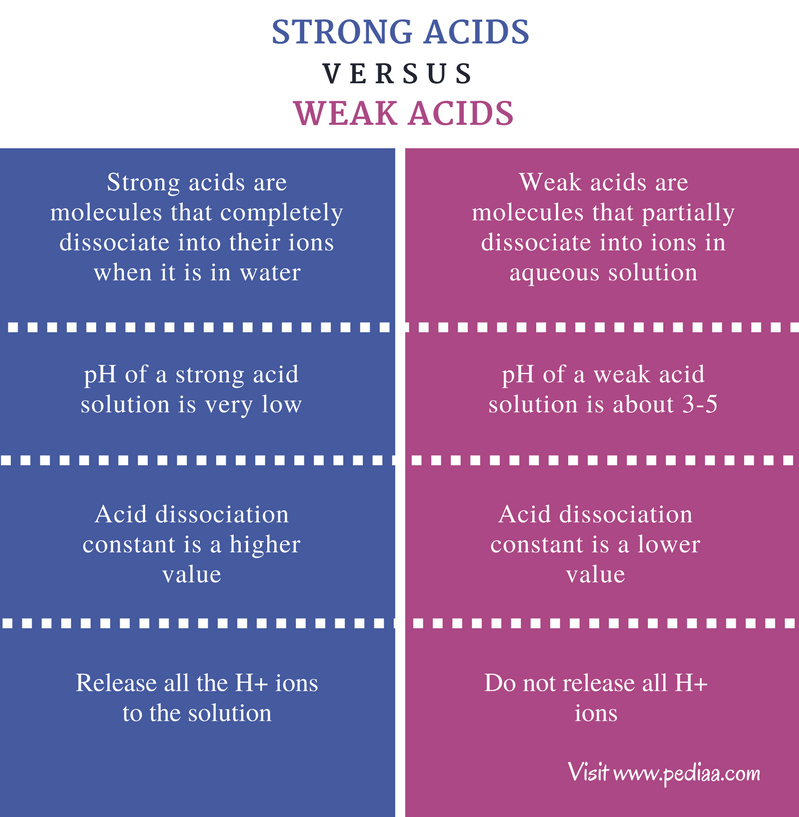
This means that the pH values of strong acids are lower than that of weak acids which explains why the rate of reaction of strong acids with substances
Accessibility our specifically curated collection of printable worksheets concentrated on What Does Weaker Acid Mean These worksheets accommodate various skill degrees, making sure a tailored knowing experience. Download and install, print, and take pleasure in hands-on activities that strengthen What Does Weaker Acid Mean skills in an effective and delightful way.
I Have A Weak Urine Stream What Could It Mean Urology Center Of

I Have A Weak Urine Stream What Could It Mean Urology Center Of
The key idea to remember is this the stronger the conjugate acid the weaker the conjugate base Sulfuric acid is the strongest acid on our list with a pK a value of 10 so HSO 4 is the weakest conjugate base
Whether you're an educator trying to find reliable approaches or a student looking for self-guided methods, this area uses sensible ideas for understanding What Does Weaker Acid Mean. Benefit from the experience and insights of instructors who focus on What Does Weaker Acid Mean education and learning.
Connect with similar individuals that share an interest for What Does Weaker Acid Mean. Our neighborhood is a room for instructors, moms and dads, and students to trade concepts, inquire, and celebrate successes in the trip of grasping the alphabet. Sign up with the conversation and be a part of our growing area.
Get More What Does Weaker Acid Mean



/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png?w=186)
https:// chem.libretexts.org /Bookshelves/General...
Stronger acids form weaker conjugate bases and weaker acids form stronger conjugate bases Thus strong acids are completely ionized in aqueous solution because their conjugate bases are weaker bases than water

https:// chem.libretexts.org /Bookshelves/Physical...
A weak acid is one which doesn t ionize fully when it is dissolved in water Ethanoic acid is a typical weak acid It reacts with water to produce hydroxonium ions and ethanoate ions but the back reaction is more successful than the forward one The ions react very easily to reform the acid and the water
Stronger acids form weaker conjugate bases and weaker acids form stronger conjugate bases Thus strong acids are completely ionized in aqueous solution because their conjugate bases are weaker bases than water
A weak acid is one which doesn t ionize fully when it is dissolved in water Ethanoic acid is a typical weak acid It reacts with water to produce hydroxonium ions and ethanoate ions but the back reaction is more successful than the forward one The ions react very easily to reform the acid and the water

Strong And Weak Acids Collection With Educational Diagram Outline

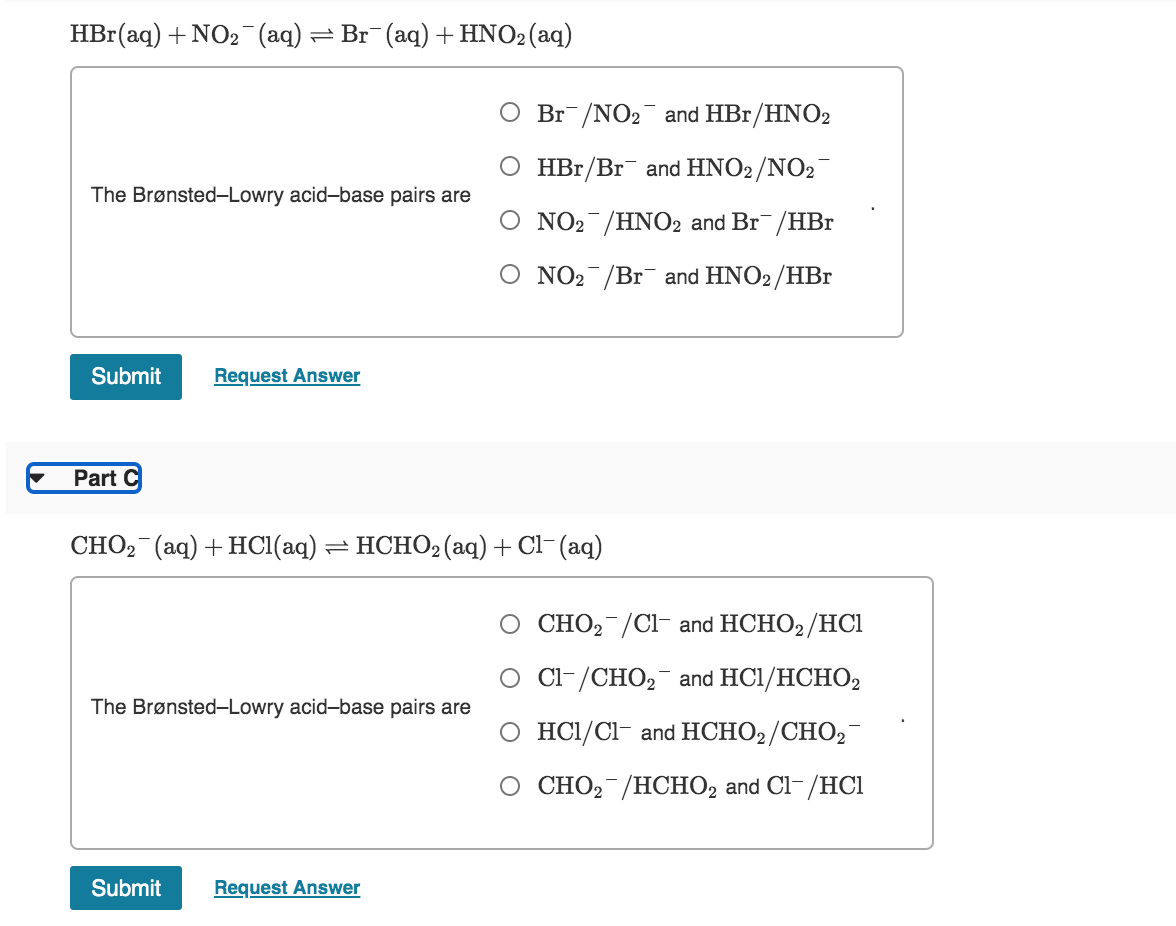
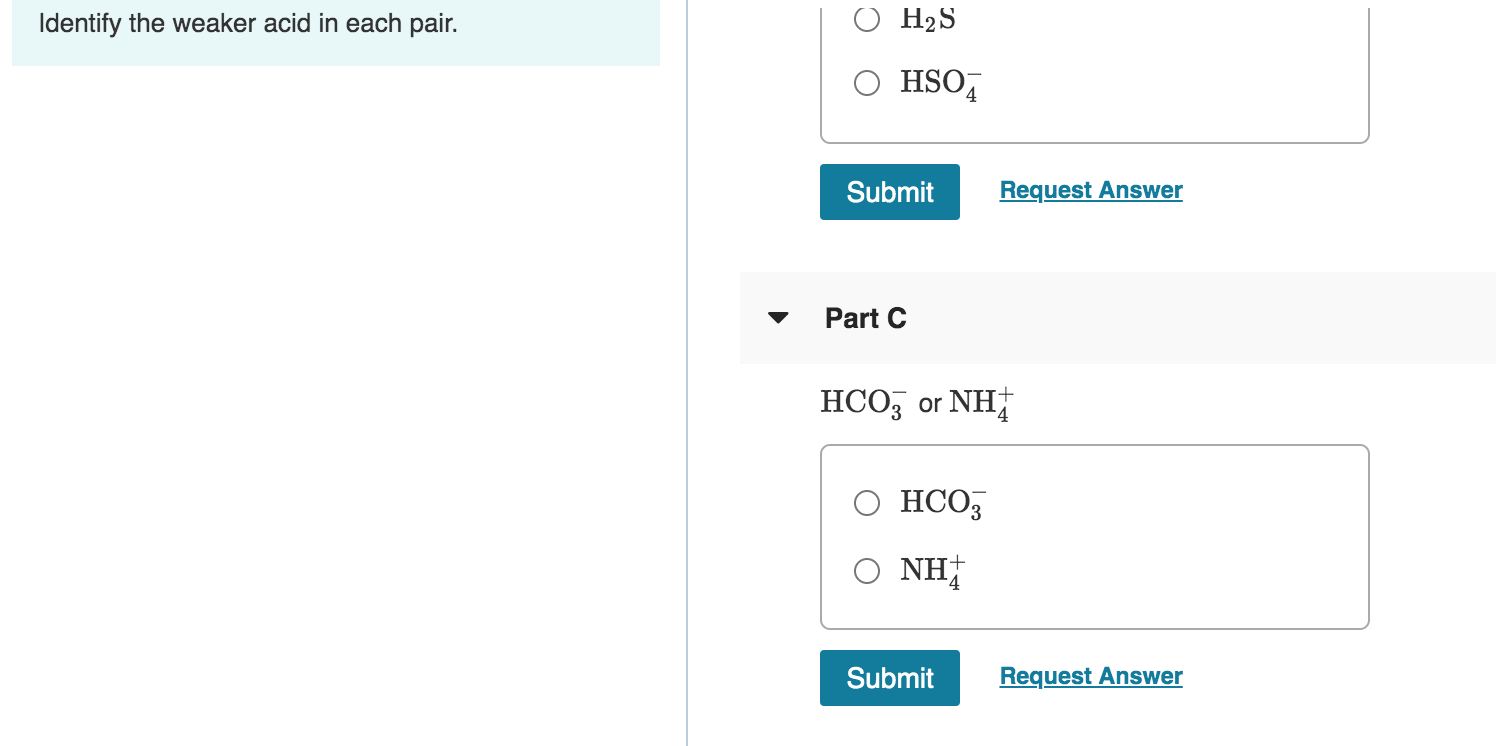
Solved 1 Review Constants Identify The Weaker Acid In Each Chegg

Difference Between Strong And Weak Acids Definition Properties Examples

Solved 1 Review Constants Identify The Weaker Acid In Each Chegg
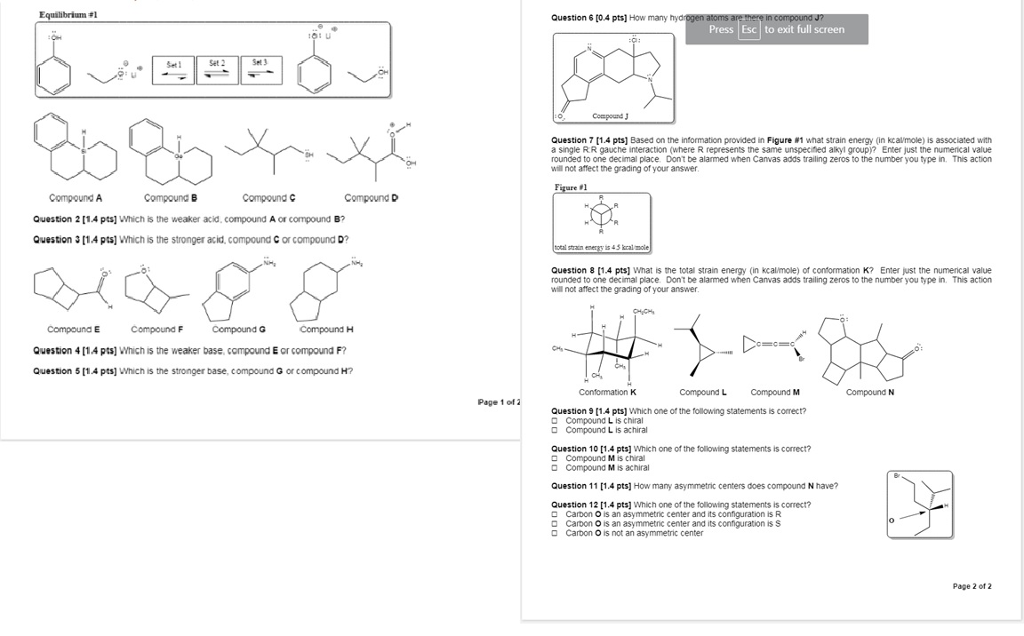
Which Is The Weaker Acid Compound A Or Compound B Chegg

Lactic Acid What Is It What Increases It And More Osmosis

Lactic Acid What Is It What Increases It And More Osmosis

What A Weaker Yuan Means For China Property