Welcome to PrintableAlphabet.net, your best resource for all things associated with Why Are Group 7 Elements Called Diatomic In this comprehensive overview, we'll look into the ins and outs of Why Are Group 7 Elements Called Diatomic, offering beneficial insights, engaging activities, and printable worksheets to enhance your understanding experience.
Recognizing Why Are Group 7 Elements Called Diatomic
In this area, we'll check out the basic concepts of Why Are Group 7 Elements Called Diatomic. Whether you're a teacher, parent, or learner, getting a strong understanding of Why Are Group 7 Elements Called Diatomic is vital for successful language procurement. Expect insights, tips, and real-world applications to make Why Are Group 7 Elements Called Diatomic come to life.
What Are The 7 Diatomic Elements
/iodine-sublimation-583678632-595d20153df78c4eb676cb4e.jpg)
Why Are Group 7 Elements Called Diatomic
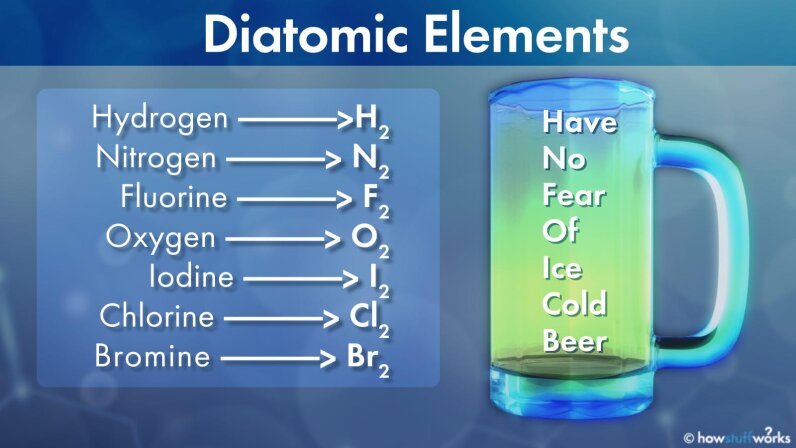
Diatomic elements are pure elements that form molecules consisting of two atoms bonded together There are seven diatomic elements hydrogen nitrogen oxygen fluorine chlorine iodine and bromine These
Discover the significance of grasping Why Are Group 7 Elements Called Diatomic in the context of language development. We'll review how proficiency in Why Are Group 7 Elements Called Diatomic lays the foundation for improved reading, creating, and general language abilities. Explore the more comprehensive impact of Why Are Group 7 Elements Called Diatomic on effective interaction.
What Are Diatomic Elements How To Remember Them
What Are Diatomic Elements How To Remember Them
Halogens are diatomic meaning they form molecules made of pairs of atoms sharing electrons forming a single covalent bond between the two halogen atoms such as F 2 C l2 etc When halogen atoms gain an electron during
Discovering doesn't need to be plain. In this section, discover a range of interesting activities tailored to Why Are Group 7 Elements Called Diatomic learners of any ages. From interactive games to creative exercises, these tasks are made to make Why Are Group 7 Elements Called Diatomic both fun and academic.
What Are The 7 Diatomic Elements Definition And List

What Are The 7 Diatomic Elements Definition And List
All of the halogens exist as diatomic molecules This means that the elements are made up of pairs of atoms that are chemically joined together for example fluorine exists as F 2
Gain access to our specifically curated collection of printable worksheets focused on Why Are Group 7 Elements Called Diatomic These worksheets cater to numerous skill levels, guaranteeing a personalized discovering experience. Download, print, and take pleasure in hands-on activities that reinforce Why Are Group 7 Elements Called Diatomic abilities in an effective and enjoyable means.
Can You Name The 7 Diatomic Elements How To Memorize Things

Can You Name The 7 Diatomic Elements How To Memorize Things
The periodic table has several diatomic elements sometimes known as molecular elements Let s learn what they are and how they are different from diatomic molecules What are the 7 Diatomic Elements The 7 diatomic
Whether you're a teacher trying to find effective methods or a student seeking self-guided strategies, this section supplies sensible tips for understanding Why Are Group 7 Elements Called Diatomic. Take advantage of the experience and understandings of teachers that specialize in Why Are Group 7 Elements Called Diatomic education and learning.
Connect with similar individuals who share a passion for Why Are Group 7 Elements Called Diatomic. Our area is a space for instructors, parents, and students to trade concepts, inquire, and celebrate successes in the journey of understanding the alphabet. Sign up with the discussion and be a part of our growing community.
Here are the Why Are Group 7 Elements Called Diatomic



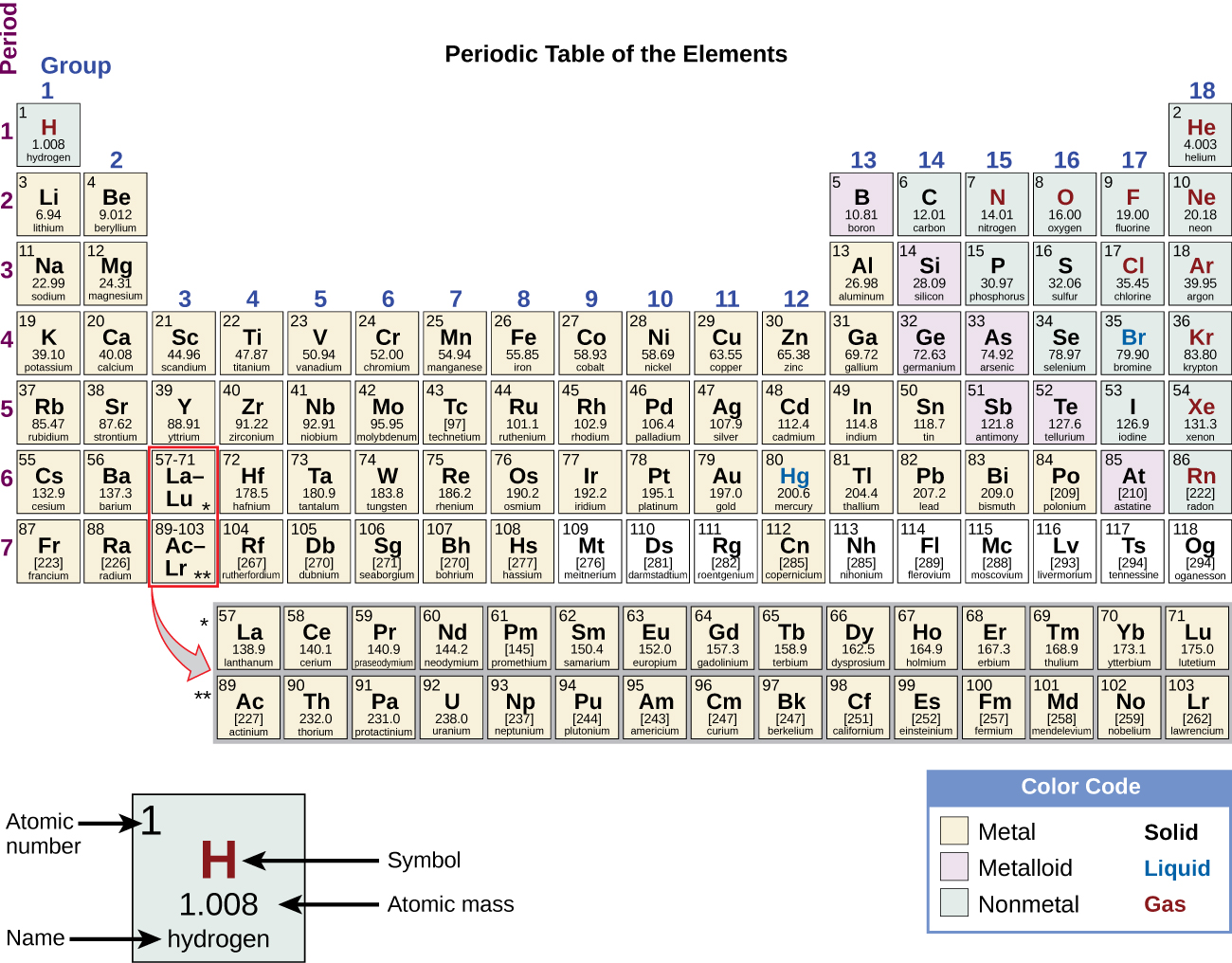

/complete-periodic-table-of-elements-royalty-free-vector-166052665-5a565f0e47c2660037ab8aca.jpg)
/iodine-sublimation-583678632-595d20153df78c4eb676cb4e.jpg?w=186)
https://www.thoughtco.com
Diatomic elements are pure elements that form molecules consisting of two atoms bonded together There are seven diatomic elements hydrogen nitrogen oxygen fluorine chlorine iodine and bromine These
https://www.savemyexams.com › ...
Halogens are diatomic meaning they form molecules made of pairs of atoms sharing electrons forming a single covalent bond between the two halogen atoms such as F 2 C l2 etc When halogen atoms gain an electron during
Diatomic elements are pure elements that form molecules consisting of two atoms bonded together There are seven diatomic elements hydrogen nitrogen oxygen fluorine chlorine iodine and bromine These
Halogens are diatomic meaning they form molecules made of pairs of atoms sharing electrons forming a single covalent bond between the two halogen atoms such as F 2 C l2 etc When halogen atoms gain an electron during

The Periodic Table Chemistry And The Environment

21 What Are The 8 Diatomic Elements What Does It Mean To Be Diatomic

Diatomic Elements Definition Example More
/complete-periodic-table-of-elements-royalty-free-vector-166052665-5a565f0e47c2660037ab8aca.jpg)
List Of Halogens Element Groups
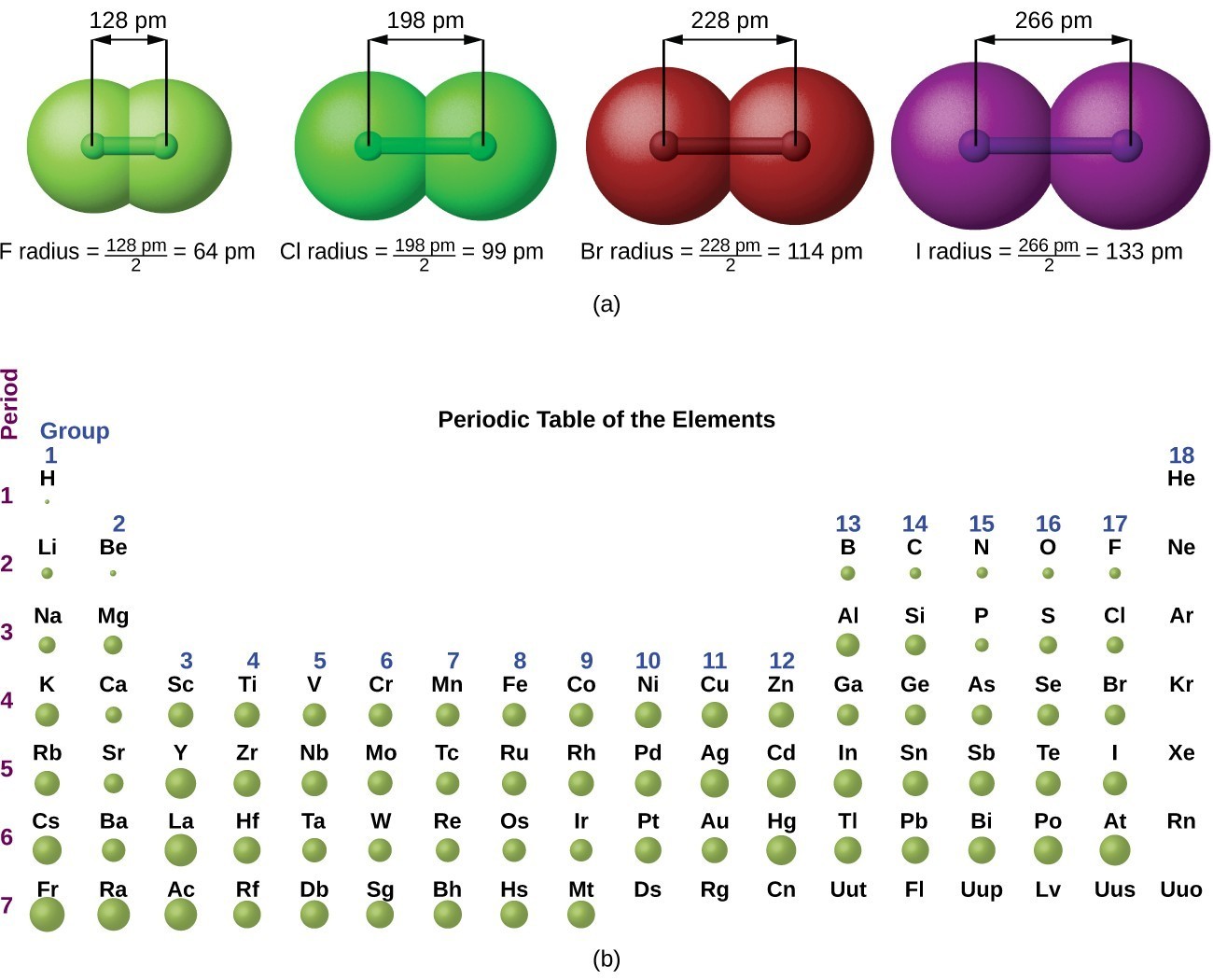
Periodic Variations In Element Properties Chemistry For Majors
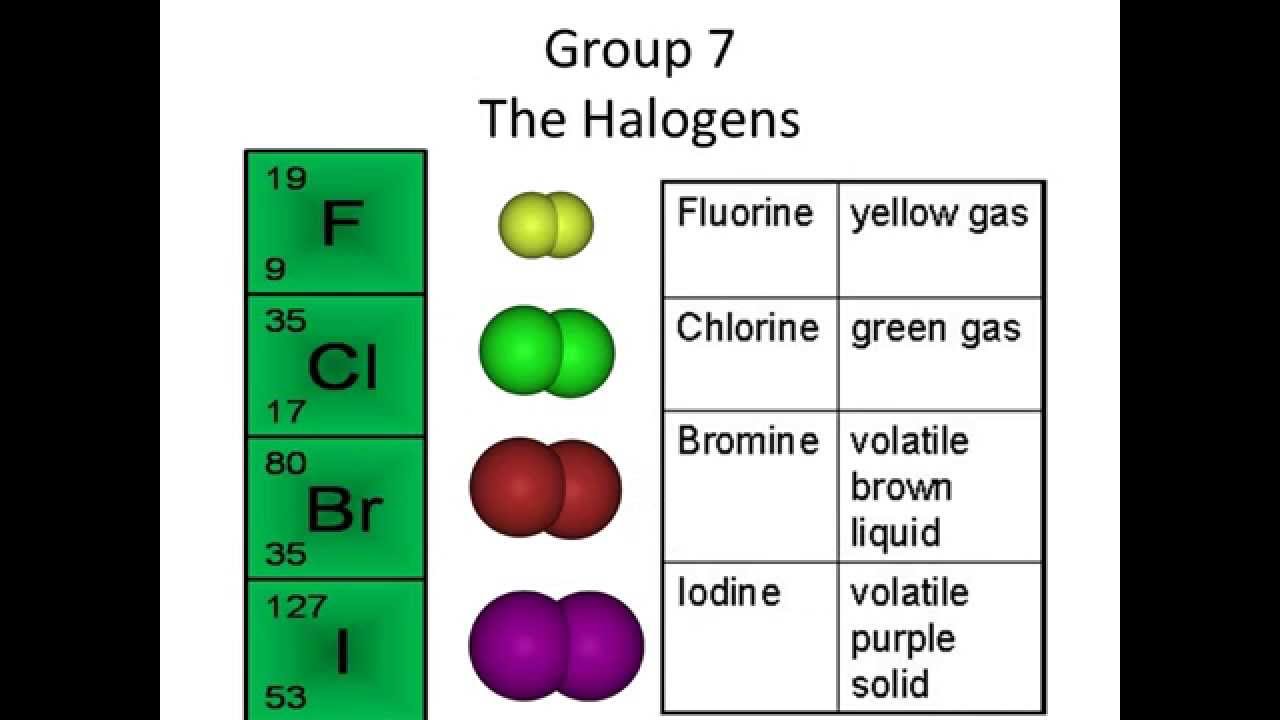
The Halogens Group 7 YouTube

The Halogens Group 7 YouTube

The Periodic Table And Its Design Pathways To Chemistry